Theme: Frontiers in the field of Pharmaceutical Education & Practice
PHARMA EDUCATION 2021
- Pharma Education 2021
- Sessions/ Tracks
- Market Analysis
- Abstract Submission Criteria & Eligibility
- Visa & Embassy
Pharma Education 2021 welcomes attendees, presenters, and exhibitors from all over the world to Vancouver, Canada. We are delighted to invite you all to attend and register for the “13th International Conference on Pharmaceutical Education & Practice” is scheduled to be held during October 29-30, 2021 at Vancouver, Canada. This Pharmaceutical Sciences Conference includes a wide range of Keynote presentations, Plenary talks, Symposia, Workshops, Exhibitions, Poster presentations and Career development programs.
The conference will be organized around the theme “Frontiers in the field of Pharmaceutical Education & Practice”. Our goal is to deliver an outstanding program which covers the entire spectrum of research & innovations in Pharma, Pharmaceutical science, Biopharmaceutics and Radiopharmaceuticals share the cross-cultural experiences of various treatment procedures.
Pharma Education 2021 is organized by Conference Series LLC Ltd. Conference series LLC Ltd Organizes 3000+ Global Events Every Year across USA, Canada, Europe & Asia with support from 1000 more scientific societies and Publishes 700+ Open access journals which contains over 100000 eminent personalities, reputed scientists as editorial board and organizing committee members. The Conference series LLC Ltd website will provide you list and details about the conferences organize worldwide.
|
Conference Name
|
Venue
|
Date
|
|
Vancouver, Canada
|
October 29-30, 2021
|
Why to Attend???
Pharma Education 2021 is a multidisciplinary program with broad participation with members from around the globe focused on learning about pharmaceutical sciences and its innovations & latest advancements. This conference provides an unique opportunity to reach the largest assemblage of participants from pharmaceutical sciences community that is from academia, pharmaceutical research entities, medical groups, pharmaceutical, biomedical and medical device industries along with related associations & societies. It also provides an opportunity to establish a scientific network between the peers belonging from both industry and academia. We cordially invite all concerned people to come to join us at our event and make it successful by your participation
Pharma Education 2021 conference is a blend of multiple disciplines where it provides the platform to discuss the topics like Drug Discover, Pharmaceutical Manufacturing, Novel Drug Delivery Systems, Nanotechnology, Radiopharmaceuticals, Clinical Trials, GMP, GCP & Regulatory Affairs, Packaging and Marketing. It will educate health care researchers about discovery, development and manufacturing of new drugs. This conference conduct presentations, distribute information, meet with current and potential scientists, make a splash with new pharmaceutical research & developments, and receive name recognition at this 2-days event. World renowned speakers and the most recent techniques, developments, the newest updates in Pharmaceutical Research are hallmarks of this conference.
Who Should Attend and Who You’ll Meet
Industry:
Directors/Senior Directors/Executive Directors, CEO’s, Presidents/Vice Presidents, Heads/Leaders, Partners, Research Associates, Research Scientist of pharmaceutical, biomedical, biopharmaceutical and medical devices industries
Leaders in:
- Drug discovery & Development
- Pharmaceutical Formulations
- Manufacturing
- Drug delivery
- Bioavailability
- Drug Analysis
- Delivery Devices
- New Products
- Process R&D
- Product Enhancement
- Nanotechnology & Nano medicine
- Pharmacology & Toxicology
- Industrial pharmacy
- Pharmaceutical regulatory affairs
- PharmacoEconomics
- Radiopharmaceuticals
Academia:
Deans, Professors/ Assistant Professors/ Associate Professor, Research Scholars, Scientists and Students related to Pharmaceutical and Medical Sciences.
Track 1: Pharmaceutical Sciences: Academic and Industry Overview
Pharmaceutical sciences combine a broad range of scientific disciplines that are involved with the design, action, delivery, disposition and use of drugs. This field draws on many areas of the basic and applied sciences such as chemistry, biology, epidemiology, statistics, chemometrics, mathematics, physics and chemical engineering and applies their principles to the study of drugs.
Track 2: Applied Pharmaceutical Sciences
Applied pharmaceutical science is a scientific discipline that applies existing scientific knowledge to develop more practical applications, such as technology or inventions. Applied pharmaceutical science applies the basic science toward practical endeavors. Applied pharmaceutical science is typically engineering, which develops technology. Applied Pharmaceutical Sciences involve in either experimental or theoretical in the following areas: Pharmaceutics & Biopharmaceutics, Novel &Targeted Drug Delivery, Nanotechnology& Nanomedicine, Pharmaceutical chemistry, Pharmacognosy & Ethnobotany Phytochemistry, Pharmacology & Toxicology, Pharmaceutical Biotechnology & Microbiology, Pharmacy practice & Hospital Pharmacy.
Track 3: Drug Discovery, Design & Development: Challenges and Approaches
Drug Discovery changes with the change in the dosage forms and the environmental conditions or demand. It involves a wide range of scientific disciplines including biology, chemistry and pharmacology. Drug design involves the design of such molecules that are similar to the bio molecular target site in shape and charge in order to bind to it. The process of bringing a new pharmaceutical drug into the market once a lead compound has been identified through the process of drug discovery is called drug development and the research related to drug development is called drug development research.
Track 4: Pharmaceutical Manufacturing
The pharmaceutical industry includes the manufacture, extraction, processing, purification and packaging of chemical materials to be used as medications for humans or animals. Pharmaceutical manufacturing is divided into two major stages: the production of the active ingredient or drug (primary processing, or manufacture) and secondary processing .The pharmaceutical Manufacturing is largely driven by scientific discovery and development, in conjunction with toxicological and clinical experience.
Track 5: Pharmaceutical Technology & Development
Pharmaceutical Development and Technology explores the research in the design, development, manufacture and evaluation of traditional and novel drug delivery systems- emphasizing practical solutions and applications to theoretical and research-based problems. Pharmaceutical products are made by transforming chemical compounds with useful effects on the human body into high-quality dosage forms that can appropriately exhibit effects against diseases. Efficacy and safety are the primary requirements for any pharmaceutical product. However, it is becoming significantly more important to provide pharmaceutical products that can be more easily used by patients, healthcare professionals and caregivers in order to respond to the rapid aging of society and the needs for advanced medical care.
Track 6: Radiopharmaceuticals
Radiopharmaceuticals are unique medicinal formulations containing radioisotopes which are used in major clinical areas for diagnosis or therapy, many radiopharmaceutical preparations contain radioisotopes with very short half-lives and such preparations therefore have very short shelf-lives and they require an expiry date and time to be indicated. Radiopharmaceutical preparation is a medicinal product in a ready-to-use form suitable for human use that contains a radionuclide. The radionuclide is integral to the medicinal application of the preparation, making it appropriate for one or more diagnostic or therapeutic applications.
Track 7: Nanotechnology in Pharmaceuticals
Nanotechnology is the science that deals with the processes that occur at molecular level and of nanolength scale size. Nanotechnology provides intelligent systems, devices and materials for better pharmaceutical applications. Pharmaceutical nanotechnology provides two basic types of nanotools, nanomaterials and nanodevices, which play a key role in realm of pharmaceutical nanotechnology and related fields. It is a combination of various fields like biophysics, bioengineering, and molecular biology and their combined disciplines. It has emerged successful in various medical fields.
Track 8: Novel Drug Delivery Systems
Novel Drug Delivery System can be a major advance for solving the problems related towards the release of the drug at specific site with specific rate. The need for delivering drugs to patients efficiently and with fewer side effects has prompted pharmaceutical companies to engage in the development of new drug delivery system. The aim of novel drug delivery System is to provide a therapeutic amount of drug to the appropriate site in the body to accomplish promptly and then maintain the desired drug concentration.
Track 9: Bioavailability and Bioequivalence Studies
Bioavailability means the rate and extent to which the active drug substance or therapeutic moiety is absorbed from a pharmaceutical form and becomes available at the site of action. For drugs intended to exhibit a systemic therapeutic effect, bioavailability can be more simply understood as the rate and extent to which a substance or its therapeutic moiety is delivered from a pharmaceutical form into the general circulation. Bioequivalence focus on the release of a drug substance from a drug product and subsequent absorption into systemic circulation. Bioequivalence studies are a surrogate marker for clinical effectiveness and safety data as it would not normally be practical to repeat clinical studies for generic products.
Track 10: Clinical Research and Clinical Trials
Clinical research as a component of medical and health research intended to produce valuable knowledge for understanding of human disease, preventing and treating illness and promoting health. Clinical research involves interactions with patients, diagnostic clinical materials or data or populations. Clinical research and its translation into preventive and clinical care. Clinical trials are research investigations in which people volunteer to test new treatments, interventions or tests as a means to prevent, detect, treat or manage various diseases or medical conditions. Clinical trials can also help to improve health care services by raising standards of treatment.
Track 11: Advanced Pharmaceutical Analysis Techniques
Pharmaceutical analytical techniques deal with the different processes to identify or quantify a substance. The components of a pharmaceutical solution or mixture or the determination of the structures of chemical compounds used in the formulation of pharmaceutical product. These pharmaceuticals may develop impurities at various stages of their development, transportation and storage which makes the pharmaceutical risky to be administered thus they must be detected and quantified. For this analytical instrumentation and methods play an important role. Variety of analytical techniques such as titrimetric, chromatographic, spectroscopic, electrophoretic and electrochemical and their corresponding methods that have been applied in the analysis of pharmaceuticals.
Track 12: Pharmacovigilance and Risk Management
The World Health Organization (WHO) defines pharmacovigilance as the science and activities relating to the detection, evaluation, understanding and prevention of adverse reactions to medicines or any other medicine-related problems. Pharmacovigilance is to protect patients and the public wherever possible and to disseminate knowledge among the relevant professional communities and to patients in order to minimise risk. Pharmacovigilance system or the processes to be engaged in risk management, there is consensus among the major regulators that pharmacovigilance is necessary and important in the development and commercialization of medicinal products.
Track 13: Challenges in GMP, GCP & Regulatory Affairs
GMP ensures that quality is built into the organization and processes involved in the manufacture of the products and all those operations should be carried out strictly according to cGMP. Current Good Manufacturing Practices, formal regulations contained in statutes and agency policies and concern the design, monitoring and control of manufacturing processes and facilities. GCP (Good Clinical Practice) is an international ethical and scientific quality standard for the performance of a clinical trial on medicinal products involving humans. GCP includes all aspects of a clinical trials. Regulatory affairs is a comparatively new profession which developed from the desire of governments to protect public health by controlling the safety and efficacy of products in areas including pharmaceuticals, veterinary medicines, medical devices, pesticides, agrochemicals, cosmetics and complementary medicines
Track 14: Pharmaceutical Packing and Marketing
Pharmaceutical Packaging can be defined as an economical means of providing presentation, protection, identification information, containment, convenience and compliance for a product during storage, carriage, display and until the product is consumed. Packaging must provide protection against climatic conditions biological, physical and chemical hazards and must be economical. Pharmaceutical marketing is based on product type and geography. The pharmaceutical packaging market is constantly advancing and has experienced annual growth of at least five percent per annum in the past few years as with most other packaged goods, pharmaceuticals need reliable and speedy packaging solutions that deliver a combination of product protection, quality, tamper evidence, patient comfort and security needs.
Track 15: Growth Strategies for Pharmaceutical Industries
Management Center Europe reports that mergers of pharmaceutical companies will contribute more than 50 percent of the industry’s future growth in the global markets. By combining their resources, pharmaceutical companies leverage their strengths to increase market share and influence. Investing in technological innovations is a profitable business strategy for a pharmaceutical business. Trends such as increasing competition, globalization and shorter product-cycle times are real challenges for the pharmaceutical industry. An operational marketing and sales strategy is paramount for a pharmaceutical company’s growth and profitability.
Track 16: Hospital Pharmacy & Industrial Pharmacy
Hospital pharmacy is a specialized field of pharmacy that is integrated into the care of a medical center. These include centers such as a hospital, outpatient clinic, drug-dependency facility, poison control center, drug information center of residential care facility. Industrial Pharmacy is a discipline which includes manufacturing, development, marketing and distribution of drug products including quality assurance of these activities. The pharmaceutical industry is an important component of health care systems throughout the world.
Track 17: Medicinal Chemistry
Significance of Recent Trends reviews the state of the art and aims to determine the significance of technology and market trends in medicinal chemistry for advancing productivity in drug discovery. Although the fundamental task of medicinal chemists has not changed drastically over time, the chemical and computational tools and perspectives at their disposal have advanced significantly. One in particular, fragment-based drug design, stands out as promising major improvements in research productivity. We examine medicinal chemistry-related approaches and methodologies that drug discovery organizations employ in an effort to increase productivity in early drug discovery and decrease attrition at later pipeline stages. Key topics considered include structure-based drug design, fragment-based drug design, natural products-based drug design, diversity-oriented synthesis, and chemogenomics. An overall assessment of the current and potential value of these approaches is presented. Various flavors of computer-aided drug design are also considered, as the complexity and limitations of drug discovery programs that are based on biochemical screens of large compound collections have been major factors in stimulating the growth of this modality.
Track 18: Process Chemistry
Process chemistry involves the manufacturing of active pharmaceutical ingredients (APIs) and key intermediates require long sequence of chemical reactions and large quantities of solvents. Production of hazardous waste as side products in these processes, poses a great threat to the environment. Therefore, the R&D’s are focusing on developing faster, cheaper, efficient and more environmentally friendly processes.
- Development of more efficient catalysts
- Developing new process technologies by using Micro reactors, Nano-encapsulation
- Exploration of various techniques for the preparation of Chiral drugs
- Developing green technologies for the manufacture of APIs and intermediates
Glance at Market of Pharmaceutical Education and Practice:
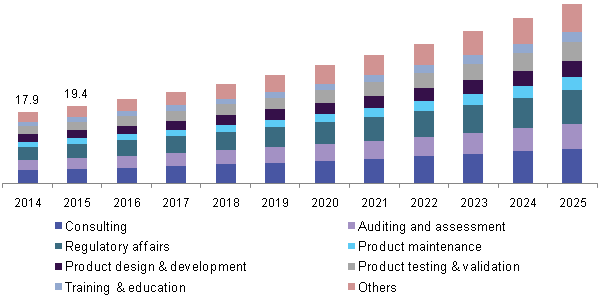
The United States is the world’s largest market for pharmaceuticals and the world leader in biopharmaceutical research.The global market for pharmaceutical Research was $70.1 billion in 2017 and $68.9 billion in 2019and this market is expected to rise at a compound annual growth rate (CAGR) of 2.3% from 2013 to 2018 and reach $77.1 billion by 2018 and the global advanced Pharma Research market should grow from roughly $178.8 billion in 2015 to nearly $227.3 billion by 2020, with a compound annual growth rate (CAGR) of 4.9%.The major attraction includes Liberty Bell, Franklin Institute, Eastern State Penitentiary, Barnes Foundation and many more.
PRESENTATION REQUIREMENTS:
- Presenting authors are responsible for registration, travel, and hotel costs. Note: Those with accepted abstracts will receive an acceptance mail allowing them to register for the conference.
- Abstracts will be compiled and conference books are made available to participants at the conference.
- Any presenter who is unable to attend should arrange for another qualified individual to present the paper/poster in question. If such a change is necessary, please notify our conference team
SUBMISSION OPTIONS:
- Oral paper presentations will have 30-minute time slots and be clustered by theme into sessions. The keynote session will have for 45-minute time slot, workshop/ special session will have 60-minute time slot and symposium will have 60-minute time slot followed by 5-minute Q&A session.
- Graduate & Masters students are eligible to submit their abstracts under poster and e-poster presentation category.
- Ph.D. students are eligible to submit their abstract under special YRF (young researcher’s forum), poster and e-poster presentation category.
NOTE: YRF category includes short oral presentation especially for Ph. D. students
- Extended abstract: Submissions should utilize the Abstract Template. Papers submitted in this category may represent original empirical research, theoretical development, reviews, or critiques.
Pharma Education 2021 organizing committee hereby reiterates that we are NOT authorized to assist with any Visa application works. You may be required to submit a Letter of Invitation, Letter of Abstract Acceptance and Registration Payment Receipt to the embassy.
Letter of Invitation: A Letter of Invitation is a proof that your paper submission and registration application are accepted by the conference committee board. It will be stated in English and may help with your visa application.
Token Amount: Token amount of USD 199 can be paid and payment receipt can be a proof for payment and may help with your VISA application.
**SHOULD YOUR APPLICATION BE DENIED, PHARMA EDUCATION 2021 ORGANIZING COMMITTEE CANNOT CHANGE THE DECISION OF THE MINISTRY OF FOREIGN AFFAIRS, NOR WILL WE ENGAGE IN DISCUSSION OR CORRESPONDENCE WITH THE MOFA OR THE EMBASSY ON BEHALF OF THE APPLICANT. THE REGISTRATION FEE WILL BE REFUNDED WHEN THE VISA APPLICATION OF INDIVIDUAL IS DENIED AND SHOULD SUBMIT VISA REJECTION PROOF. T&C APPLY**
Conference Highlights
- Pharmaceutical Technology & Development
- Advanced Pharmaceutical Analysis Techniques
- Pharmacovigilance and Risk Management
- Challenges in GMP, GCP & Regulatory Affairs
- Pharmaceutical Packing and Marketing
- Growth Strategies for Pharmaceutical Industries
- Hospital Pharmacy & Industrial Pharmacy
- Medicinal Chemistry
- Process Chemistry
- Radiopharmaceuticals
- Nanotechnology in Pharmaceuticals
- Novel Drug Delivery Systems
- Pharmaceutical Sciences: Academic and Industry Overview
- Bioavailability and Bioequivalence Studies
- Clinical Research and Clinical Trials
- Applied Pharmaceutical Sciences
- Drug Discovery, Design & Development: Challenges and Approaches
- Pharmaceutical Manufacturing
- Pharmaceutical Education and Practice System
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | October 29-30, 2021 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmaceutical Sciences & Emerging Drugs .
- Journal of Pharmaceutics & Drug Delivery Research .
Abstracts will be provided with Digital Object Identifier by